| Biotin | ||
| Biotin is water-soluble and is required by all organisms. In human intestine, bacteria produce biotin.
Recommended daily intake: 30 µg |
||
Biotin is present in the skin, hair, nerves and bone marrow.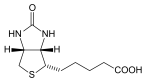 Biotin deficiency rarely, if ever, occurs in healthy individuals who consume a regular diet. Almost all foods contain significant quantities of biotin, and many widely consumed foods are relatively rich in biotin. the intestinal flora synthesizes significant quantities of biotin. A significant fraction of the body’s biotin is recycled; that is, a given molecule of biotin may be repeatedly used before it is eventually lost from the body in the feces or urine.It can happen in those being treated either with certain anticonvulsants or with broad-spectrum antibiotics. Consuming raw egg white may cause biotin deficits. Avidin, a protein found in egg white, can bind biotin and prevent it being absorbed. Biotin-Avidin complex is not broken during the passage of food in the intestine and is lost in the feces. |
||
| Athletes often take biotin because they are most likely to experience a deficit. Anticonvulsants inhibit biotin absorption in the small intestine or increase urinary excretion of the vitamin. Biotin deficiency is relatively common in pregnant women, because excretion levels are higher. Pregnant women are advised to take addition biotin (at least 400 µg/ day). | ||
| Biotin deficiency causes skin, nail and hair loss. It may also result in weakness, depression, hallucination, numbness, fatigue, irritation, rashes, loss of appetite, and even depression. | ||
Symptoms of Biotin deficiency first start with skin and hair.
Approximately 1-2 weeks later, neurologic symptoms begin to develop.
Other symptoms
|
||
| Biotin is found in various food stuffs in generally lower amounts than other water-soluble vitamins. Biotin containing food products include bread, brown rice, bran cereals, egg yolk, yeast, nuts, beans, peas, cauliflower, liver, kidney and fish. Biotin supplements may be recommended in case of skin, nail and hair loss. | ||
 |
 |
 |
 |
 |
 |
 |
 |
 |
| Nutrition | ||
Phosgene
| Phosgene |
Phosgene is the chemical compound with the formula COCl2. It is a colorless gas which gained importance as a chemical weapon during World War I.Phosgene was synthesized by the British chemist John Davy in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight. as a chemical weapon during World War I.Phosgene was synthesized by the British chemist John Davy in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight.
In low concentrations, its odor resembles freshly cut hay or grass. |
| It is also an industrial reagent in synthesis of pharmaceuticals and other organic compounds. In addition to its industrial production, small amounts occur naturally from the breakdown and the combustion of organochlorine compounds, such as those used in refrigeration systems.Phosgene is produced by passing purified carbon monoxide and chlorine gas through a bed of porous activated carbon, which serves as a catalyst.
Upon ultraviolet (UV) radiation in the presence of oxygen, chloroform slowly converts into phosgene. To suppress this photodegradation, chloroform is often stored in brown-tinted glass containers. The great majority of phosgene is used in the production of isocyanates, The isocyanates are precursors to polyurethanes. Significant amounts are also used in the production of polycarbonates. Polycarbonates are an important class of engineering thermoplastic found, for example, in lenses in eye glasses. |
| Phosgene is an insidious poison as the odor may not be noticed and symptoms may be slow to appear. Its high toxicity arises from the action of the phosgene on the proteins in the pulmonary alveoli, which are the site of gas exchange. The damage to the alveoli disrupts the blood-air barrier, causing suffocation.Sodium bicarbonate may be used to neutralise liquid spills of phosgene. Gaseous spills may be neutraised with ammonia. |
| Following the extensive use of phosgene gas in combat during World War I, it was stockpiled by various countries as part of their secret chemical weapons programs.Phosgene was frequently used by the Imperial Japanese Army against the Chinese during the Second Sino-Japanese War. |
| Chemical Weapons |
Tear gas
| Tear gas |
| Among the first uses of chemicals as weapons, Tear inducing agents were used.During the first World War, the French army were the first to employ gas, using 26 mm grenades filled with tear gas (ethyl bromoacetate) in August 1914. The small quantities of tear gas used were not even detected by the Germans. Active agent was later changes to chloroacetone.In October 1914, German troops fired fragmentation shells filled with a chemical irritant against British positions at Neuve Chapelle.
These days a type of Tear Gas is routinely used by police to disperse rowdy crowd of demonstrators. |
| Chemical Weapons |
Chlorine
| Chlorine |
Chlorine was Discovered in 1774 by Carl Wilhelm Scheele.Chlorine gas is two and one half times as heavy as air, has an intensely disagreeable suffocating odor, and is exceedingly poisonous. In its liquid and solid form it is a powerful oxidizing, bleaching, and disinfecting agent.In nature it is only found combined with other elements chiefly sodium in the form of common salt – NaClIt is an essential microutrient for higher plants. Growth suffers if the amount of chloride in the soil fall below 2 ppm. air, has an intensely disagreeable suffocating odor, and is exceedingly poisonous. In its liquid and solid form it is a powerful oxidizing, bleaching, and disinfecting agent.In nature it is only found combined with other elements chiefly sodium in the form of common salt – NaClIt is an essential microutrient for higher plants. Growth suffers if the amount of chloride in the soil fall below 2 ppm. |
Chlorine is an important chemical in water purification. Chlorine is also used widely in the manufacture of many products and items directly or indirectly:
|
| Exposure to chlorine can occur in the workplace. People who use laundry bleach and swimming pool chemicals containing chlorine products are usually not exposed to chlorine itself. Chlorine is generally found only in industrial settings.Chlorine enters the body breathed in with contaminated air or when consumed with contaminated food or water. It does not remain in the body, due to its reactivity.Effects of chlorine on human health depend on how the amount of chlorine that is present, and the length and frequency of exposure. Effects also depend on the health of a person or condition of the environment when exposure occurs.Breathing small amounts of chlorine for short periods of time adversely affects the human respiratory system. Effects differ from coughing and chest pain, to water retention in the lungs. Chlorine irritates the skin, the eyes, and the respiratory system. These effects are not likely to occur at levels of chlorine that are normally found in the environment. |
|
As a Chemical Weapon The German Army first used chlorine gas cylinders in April 1915 against the French Army at Ypres. French soldiers reported seeing yellow-green clouds drifting slowly towards the Allied trenches. They also noticed its distinctive smell which was like a mixture of pineapple and pepper. At first the French officers assumed that the German infantry were advancing behind a smoke screen and orders were given to prepare for an armed attack. When the gas arrived at the Allied front-trenches soldiers began to complain about pains in the chests and a burning sensation in their throats. Chlorine gas destroyed the respiratory organs of its victims and this led to a slow death by asphyxiation. |
| Chemical Weapons |
Recommended daily intake of vitamins and minerals
Recommended daily intake of vitamins and minerals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||