|
Glucosamine |
Glucosamine is a substance that is needed for the making of the cartilage in our body. It is manufactured in our body from glucose and an amine. Its main role in the body is in the joints where it stimulates manufacture of glycosaminoglycan which is a main structural component of cartilage.
It is understood that as some people age they lose the ability to manufacture sufficient amount of glucosamine. The result is that synthesis of glycosaminoglycan does not keep up with the degradation process in the cartilages of the joints. The inability to manufacture glucosamine in adequate rate has been suggested to be the major factor leading to osteoarthritis.
Sources of Glucosamine: There are no food sources of glucosamine and the commercially available glucosamine is derived from chitin – the exoskeleton of shrimp, lobsters and crabs.
It is thought that a deficiency of glucosamine is a major factor in the development of osteoarthritis. The weight bearing joints like knees and hips and the joints of hands are the ones most affected by the osteoarthritis. In the affected joints there is substantial cartilage destruction followed by hardening and formation of large bone spurs in the joint margins.
All this results to pain, deformity and limitation of joint motion. The onset of osteoarthritis is very subtle. Morning joint stiffness is often the first symptom. As the disease progresses, there is pain on motion of the involved joint that is made worse by prolonged activity and relieved by rest.
Glucosamine is available commercially in three forms – glucosamine sulphate, glucosamine hydrochloride and N-acetyle-glucosamine. The sulphate form has been subjected to more than 300 scientific investigations. Glucosamine sulphate has also been used by millions of people worldwide and is registered as an aid in osteoarthritis in over 70 countries.
In various studies it has been shown that glucosamine sulphate produces much better results compared with other anti inflammatory medicines in the treatment of Osteoarthritis.
Side effects: Glucosamine has shown to have very few side effects. These include gastro intestinal symptoms like stomach upset, heartburn, diarrhoea, nausea and indigestion. If the symptoms occur it should be take with meals.
Glucosamine in combination with MSM (Methyl Sulphonyl Methane) is shown to have beneficial effect in the treatment of osteoarthritis.

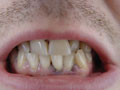 ability to concentrate.Encephalopathy is rare. A “lead line” sometimes appears at the gingiva-tooth border after prolonged high-level exposure.Chronic subclinical lead exposure is associated with interstitial nephritis, tubular damage (with tubular inclusion bodies), hyperuricemia (with an increased risk of gout), and a decline in glomerular filtration rate and chronic renal failure.An additional issue for both children and adults is whether lead that has accumulated in bone and lain dormant for years can pose a threat later in life, particularly at times of increased bone resorption such as pregnancy, lactation, and senile osteoporosis. Elevation of the bone lead level appears to be a risk factor for anemia and hypertension. Hyperthyroidism has been reported to cause lead toxicity in adults by mobilizing stores of bone lead acquired during childhood.
ability to concentrate.Encephalopathy is rare. A “lead line” sometimes appears at the gingiva-tooth border after prolonged high-level exposure.Chronic subclinical lead exposure is associated with interstitial nephritis, tubular damage (with tubular inclusion bodies), hyperuricemia (with an increased risk of gout), and a decline in glomerular filtration rate and chronic renal failure.An additional issue for both children and adults is whether lead that has accumulated in bone and lain dormant for years can pose a threat later in life, particularly at times of increased bone resorption such as pregnancy, lactation, and senile osteoporosis. Elevation of the bone lead level appears to be a risk factor for anemia and hypertension. Hyperthyroidism has been reported to cause lead toxicity in adults by mobilizing stores of bone lead acquired during childhood. 1.9 umol/L (40 ug/dL) is advised.Lead-associated anemia is usually normocytic and normochromicand may be accompanied by basophilic stippling. Lead-induced peripheral demyelination is reflected by prolonged nerve conduction time and subsequent paralysis, usually of the extensor muscles of the hands and feet –wrist and foot drop. An increased density at the metaphyseal plate of growing long bones (“lead lines”) can develop in children and
1.9 umol/L (40 ug/dL) is advised.Lead-associated anemia is usually normocytic and normochromicand may be accompanied by basophilic stippling. Lead-induced peripheral demyelination is reflected by prolonged nerve conduction time and subsequent paralysis, usually of the extensor muscles of the hands and feet –wrist and foot drop. An increased density at the metaphyseal plate of growing long bones (“lead lines”) can develop in children and resemble those seen in rickets. Children with high-level lead exposure sometimes develop Fanconi’s syndrome, pyuria, and azotemia.Adults chronically exposed to lead can develop elevated serum creatinine levels, decreased creatinine clearance rates, and chronic changes and intranuclear inclusion bodies (detected at renal biopsy).
resemble those seen in rickets. Children with high-level lead exposure sometimes develop Fanconi’s syndrome, pyuria, and azotemia.Adults chronically exposed to lead can develop elevated serum creatinine levels, decreased creatinine clearance rates, and chronic changes and intranuclear inclusion bodies (detected at renal biopsy). 
 inflammation and necrosis of the intestinal mucosa; these changes manifest as hemorrhagic gastroenteritis, fluid loss, and hypotension. Delayed cardiomyopathy accompanied by electrocardiographic abnormalities may develop. Symptoms include nausea, vomiting, diarrhea, abdominal pain, delirium, coma, and seizures. A garlicky odor may be detectable on the breath. Acute tubular necrosis and hemolysis may develop. The reported lethal dose of arsenic ranges from 120 to 200 mg in adults and is 2 mg/kg in children. Arsine gas causes severe hemolysis within 3 to 4 h of exposure and can lead to acute tubular necrosis and renal failure.In chronic arsenic poisoning, the onset of symptoms comes at 2 to 8 weeks. Typical findings are skin and nail changes, such as hyperkeratosis, hyperpigmentation, exfoliative dermatitis, and Mees’ lines (transverse white striae of the fingernails); sensory and motor polyneuritis manifesting as numbness and tingling in a “stocking-glove” distribution, distal weakness, and quadriplegia; and inflammation of the respiratory mucosa.Epidemiologic evidence has linked chronic
inflammation and necrosis of the intestinal mucosa; these changes manifest as hemorrhagic gastroenteritis, fluid loss, and hypotension. Delayed cardiomyopathy accompanied by electrocardiographic abnormalities may develop. Symptoms include nausea, vomiting, diarrhea, abdominal pain, delirium, coma, and seizures. A garlicky odor may be detectable on the breath. Acute tubular necrosis and hemolysis may develop. The reported lethal dose of arsenic ranges from 120 to 200 mg in adults and is 2 mg/kg in children. Arsine gas causes severe hemolysis within 3 to 4 h of exposure and can lead to acute tubular necrosis and renal failure.In chronic arsenic poisoning, the onset of symptoms comes at 2 to 8 weeks. Typical findings are skin and nail changes, such as hyperkeratosis, hyperpigmentation, exfoliative dermatitis, and Mees’ lines (transverse white striae of the fingernails); sensory and motor polyneuritis manifesting as numbness and tingling in a “stocking-glove” distribution, distal weakness, and quadriplegia; and inflammation of the respiratory mucosa.Epidemiologic evidence has linked chronic consumption of water containing arsenic at concentrations in the range of 10 to 1820 ppb with vasospasm and peripheral vascular insufficiency culminating in “blackfoot disease – a gangrenous condition affecting the extremities.Chronic arsenic exposure has also been associated with a greatly elevated risk of skin cancer and possibly of cancers of the lung, liver (angiosarcoma), bladder, kidney, and colon.
consumption of water containing arsenic at concentrations in the range of 10 to 1820 ppb with vasospasm and peripheral vascular insufficiency culminating in “blackfoot disease – a gangrenous condition affecting the extremities.Chronic arsenic exposure has also been associated with a greatly elevated risk of skin cancer and possibly of cancers of the lung, liver (angiosarcoma), bladder, kidney, and colon.



 Almond oil is used for skin massage. It can be used for falling hair. To improve complexion equal amount of honey and almond oil can be applied on the face.
Almond oil is used for skin massage. It can be used for falling hair. To improve complexion equal amount of honey and almond oil can be applied on the face.